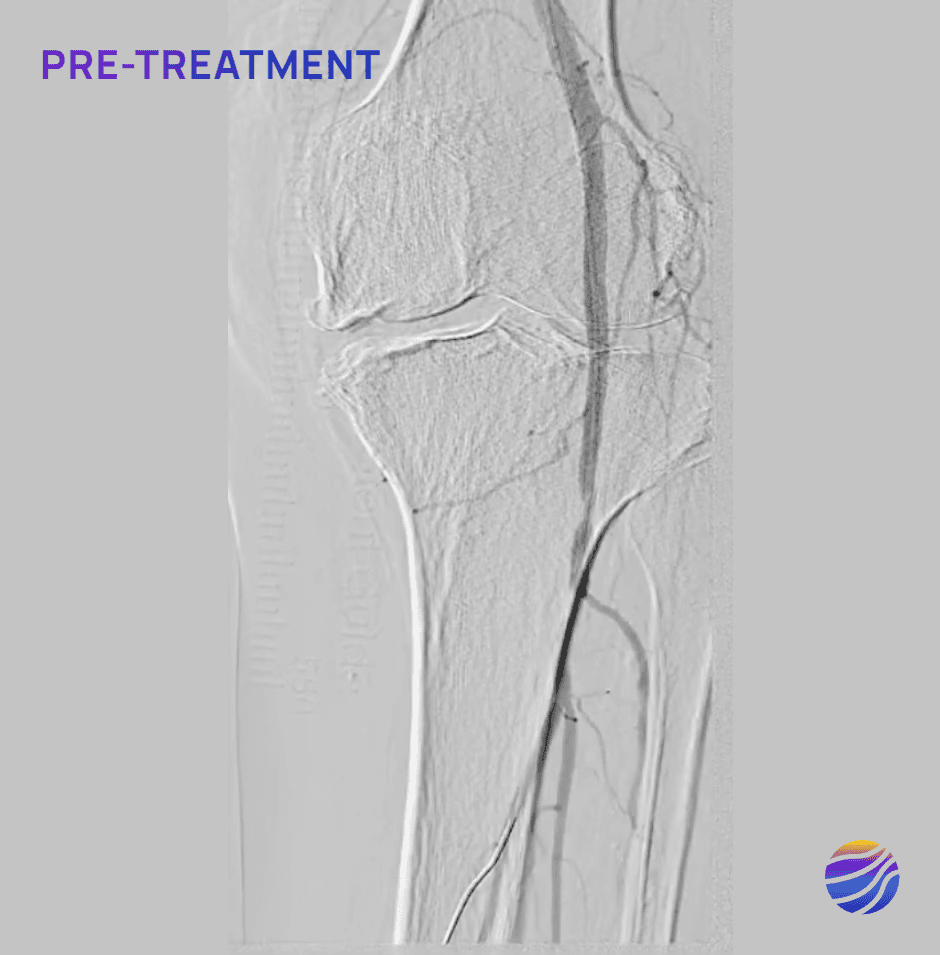
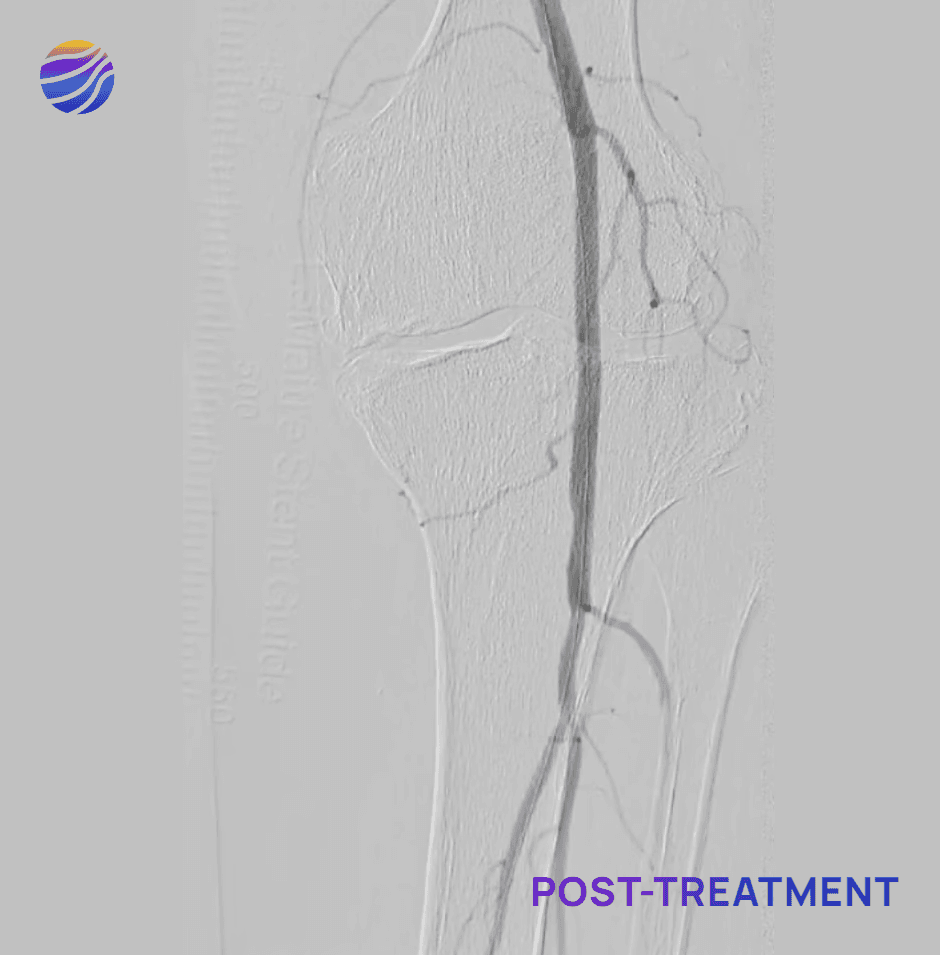
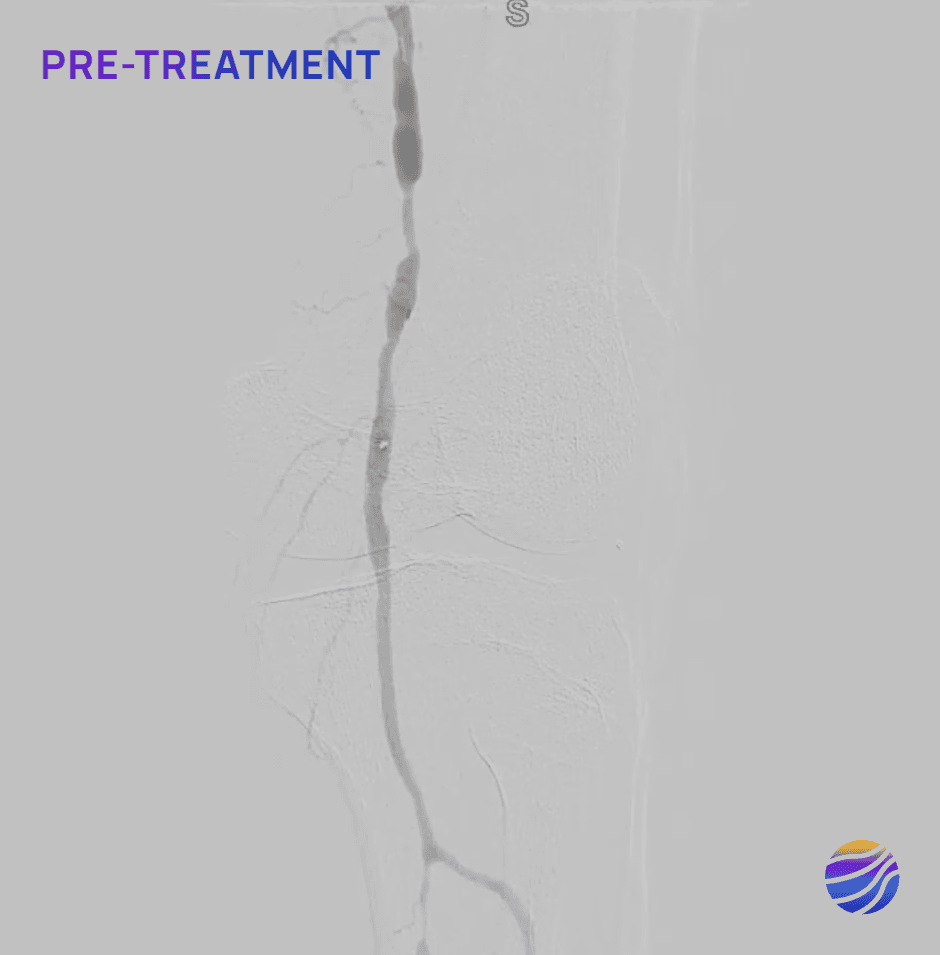
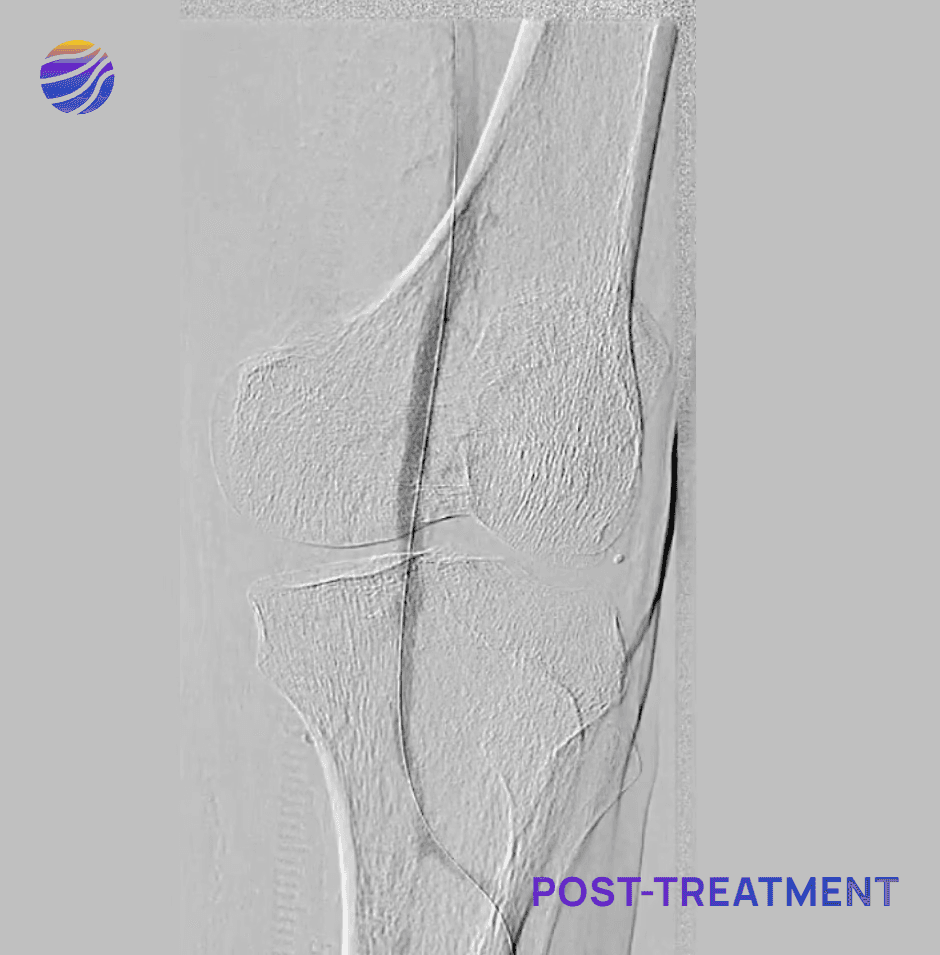
68-year-old male, BMI of 30.3, and RCC 6
Lesion Locations: Fem-Pop
Target Lesions: 3
IVL Pulses Delivered: 300
Total Lesion Length: 60mm
Results: <10% RDS
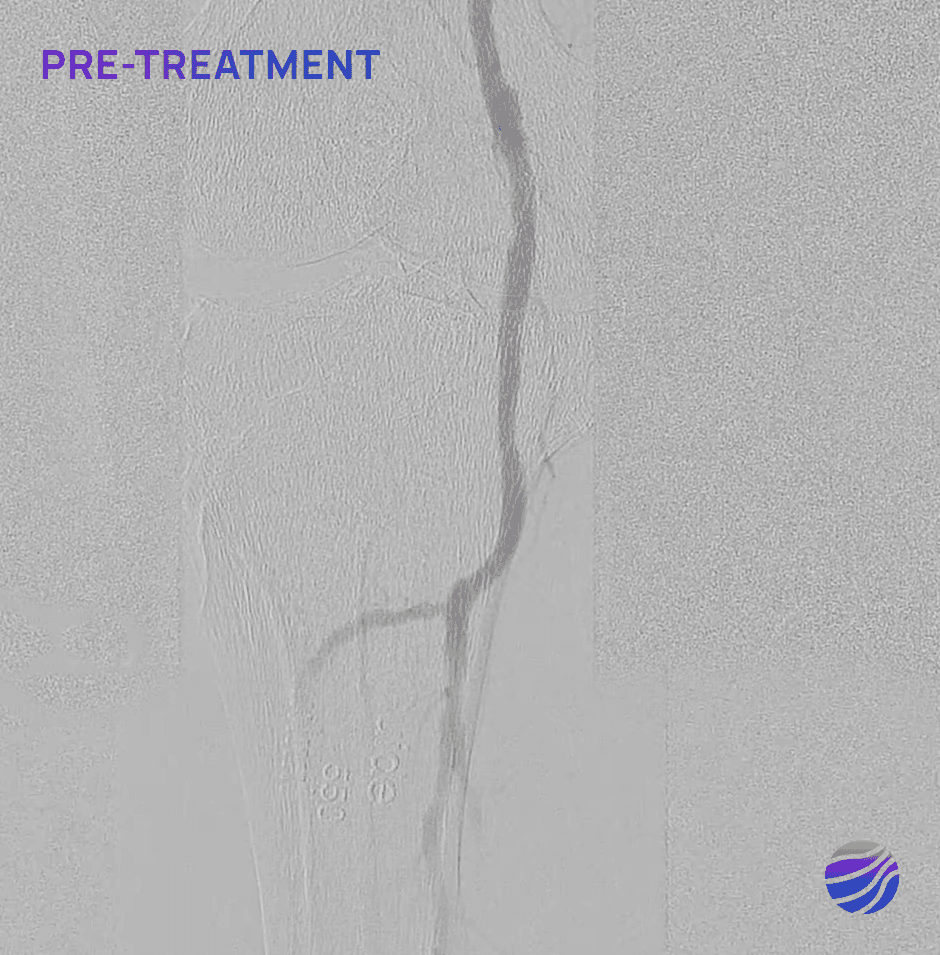
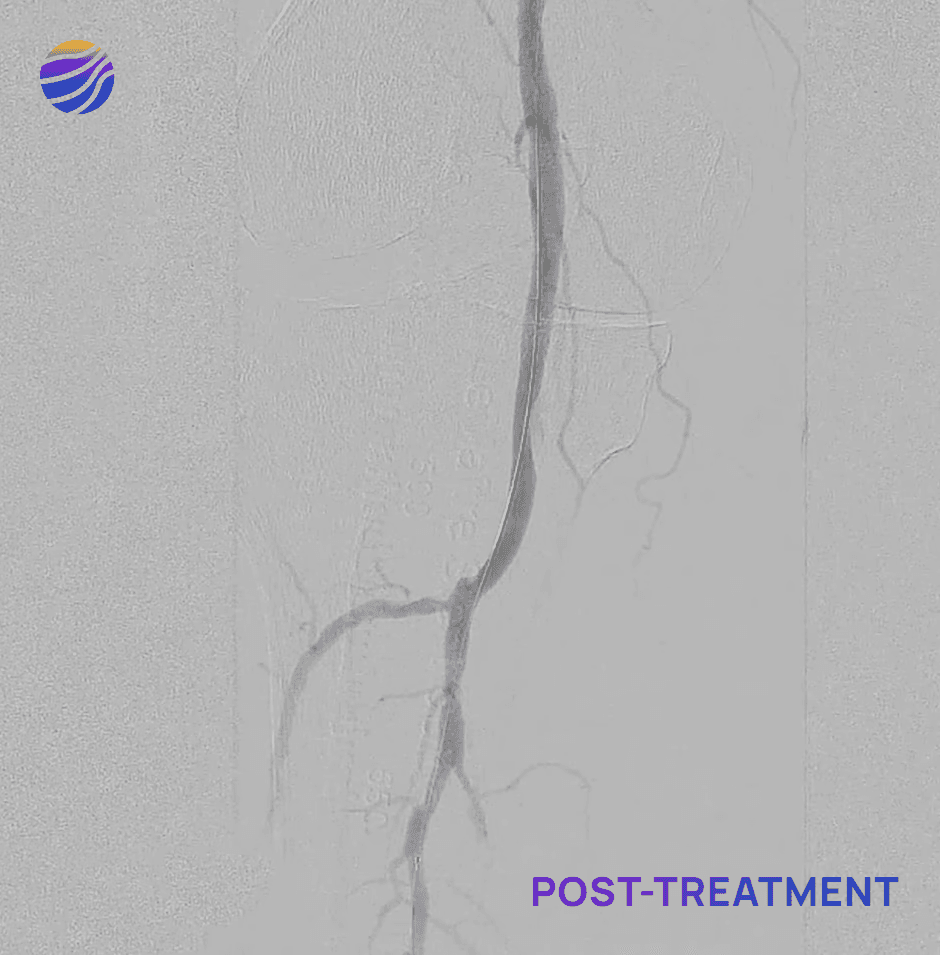
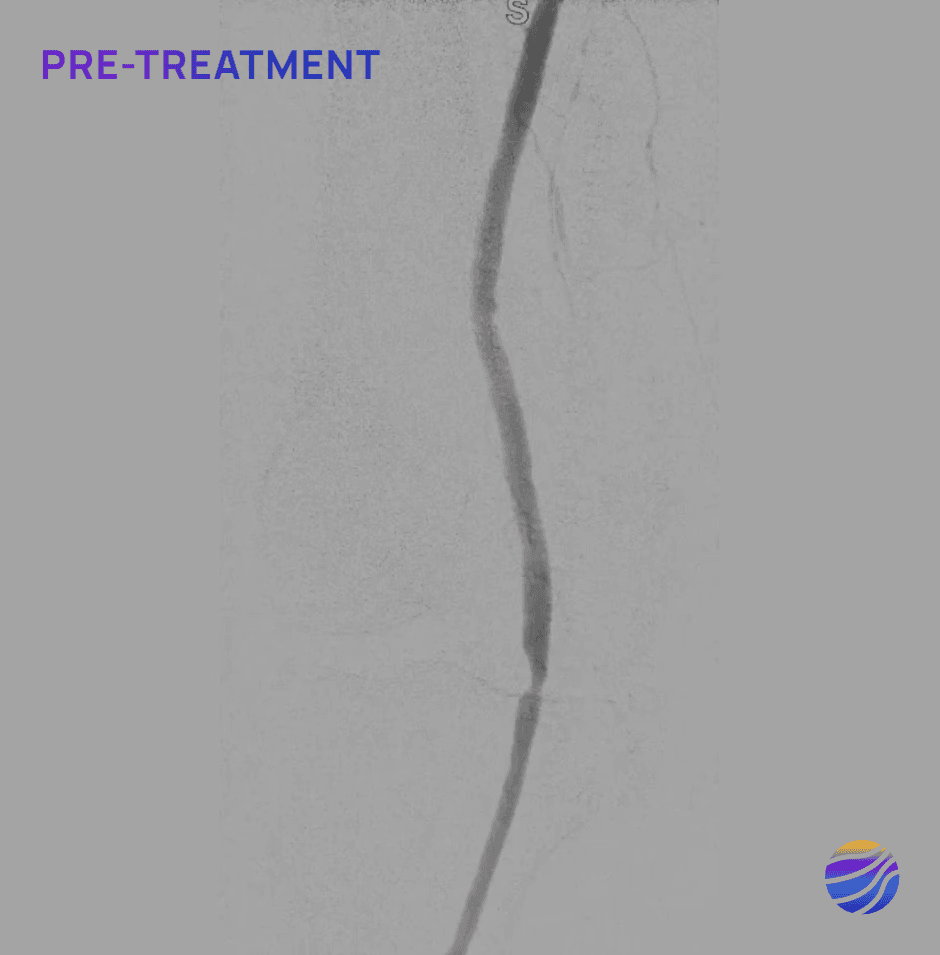
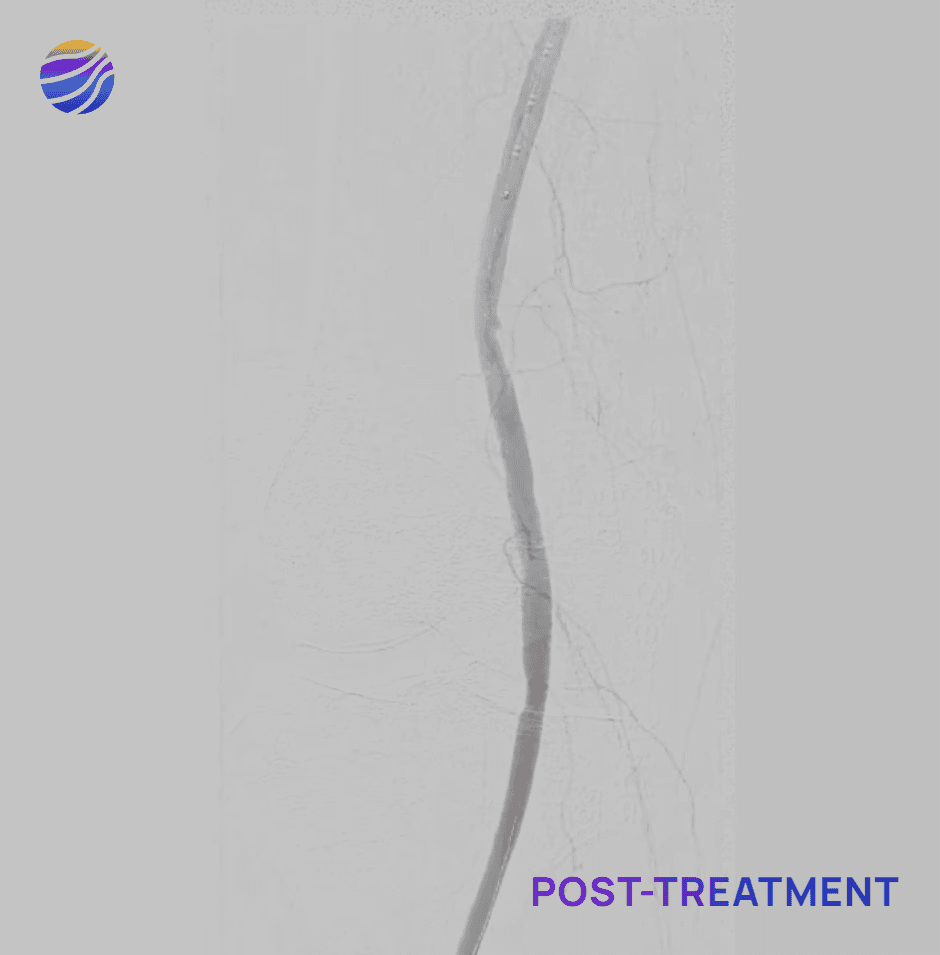
84-year-old female, BMI of 28.6, and RCC 4
Lesion Locations: Popliteal
Target Lesions: 1
IVL Pulses Delivered: 120
Total Lesion Length: 40mm
Results: 0% RDS
89-year-old male, BMI of 22.2, and RCC 4
Lesion Locations: Femoral
Target Lesions: 2
IVL Pulses Delivered: 90
Total Lesion Length: 160mm
Results: <10% RDS
71-year-old male, BMI of 27.4, and RCC 3
Lesion Locations: Fem-Pop
Target Lesions: 2
IVL Pulses Delivered: 300
Total Lesion Length: 40mm
Results: <10% RDS
Our novel coronary system has demonstrated impressive efficacy in cadaveric artery disease models, leading to compelling luminal gain through comprehensive calcium fracturing.
We anticipate IDE approval from FDA to begin our peripheral pivotal trial in the first half of 2025. This study will evaluate the safety and efficacy of our peripheral IVL system in treating patients with severely calcified ATK and BTK peripheral arterial disease.
Submit Your Site
We anticipate IDE approval from FDA to begin a coronary IDE trial in the back half of 2025. This study will evaluate the safety and efficacy of our coronary IVL system in treating patients with calcified coronary arterial disease.
Submit Your Site