FastWave: The Next Generation of Intravascular Lithotripsy (IVL)
Led by proven medtech entrepreneurs, we’re developing two differentiated IVL energy systems to advance the treatment of calcific disease.
1 in 4 deaths in the U.S. is caused by CAD.¹
Every year, CAD causes 17.8 million deaths globally.¹
A limb is amputated every 3-5 minutes in the U.S.²
1 in 2 patients die from amputation within 1 year.³
"We continue to encounter coronary and peripheral disease complicated by severe calcium."
Dr. Amir Kaki
Interventional Cardiologist
"Calcium poses significant therapeutic challenges in treating peripheral artery disease."
Dr. Venkatesh Ramaiah
Vascular Surgeron
IVL Has Been Rapidly Adopted for Calcific Disease Treatment
The utilization of IVL for percutaneous coronary interventions is expected to grow at a 49% annual compounded rate due to its ease of use, favorable safety profile, and exceptional therapeutic efficacy.⁵
Overview
Investigators
Dr. Miguel Montero-Baker, Venkatesh Ramaiah, and Antonio Muñoa
Location
San Lucas Hospital, Chiapas, Mexico
Dates
January 11-12, 2024
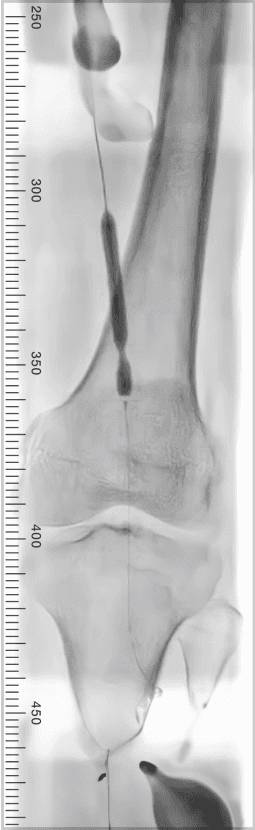
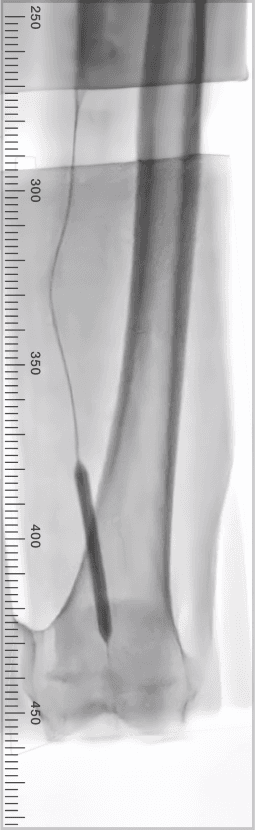
Results
9 successfully treated limbs across 8 patients
100% of lesions crossed, including 3 CTOs
Post-treatment mean residual diameter stenosis of 5.9%
0% AEs with no balloon ruptures
FastWave is Advancing at an
Unparalleled Pace
February 2021
FastWave established as a stand-alone Delaware C-corp.
August 2021
FastWave raises $12M Series A round of financing.
November 2022
USPTO grants FastWave its first utility patent within 6 months of filing.
April 2023
USPTO grants FastWave its second IVL utility patent.
June 2023
FastWave closes oversubscribed private financing round within a few weeks.
January 2024
FastWave completes enrollment for the first-in-human study of its peripheral IVL system.
March, April, and May 2024
USPTO grants FastWave its third, fourth, and fifth IVL utility patents.
June 2024
FastWave closes oversubscribed $19M financing round in less than a month.
December 2024
USPTO grants FastWave its sixth IVL utility patent.
January 2025