Seamless Usability
One-click activation. Real-time feedback. Hands-free operation. No connector cables. Artero™ is peripheral IVL streamlined — so interventionalists can focus on patients, not clunky cables.
Advanced Crossing Capabilities
Artero™ is designed to navigate challenging anatomy with a reduced crossing profile and optimized shaft for superior pushability. Our rupture-resistant balloon delivers consistent results, even in complex vessels.
Efficient and Predictable Therapy
The novel emitter design of Artero™ delivers sonic pressure at 4 Hz — twice as fast as current IVL technologies. With more pulses per catheter, experience predictable outcomes with consistent longitudinal and circumferential energy.
9
successfully treated limbs
16
target femoral-popliteal lesions
99%
moderate to severe calcification
100%
lesions crossed, including 3 CTOs
5.9%
mean residual diameter stenosis
0%
major adverse events with no balloon ruptures
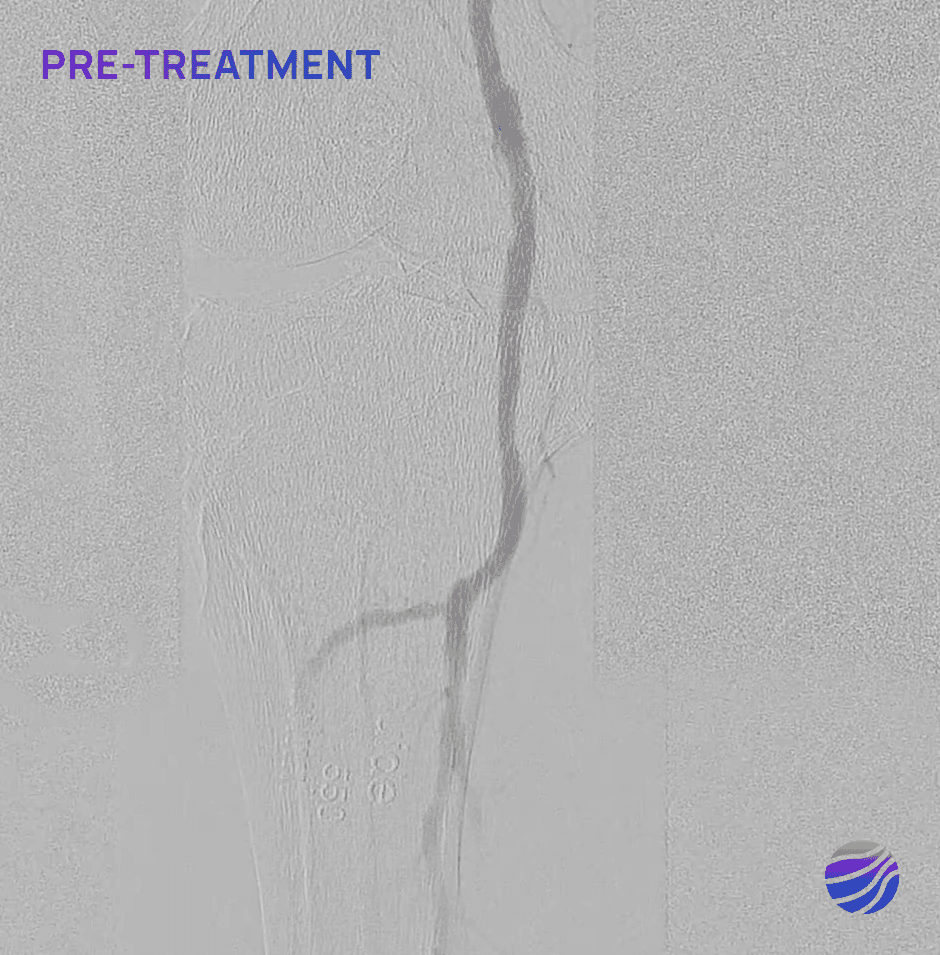
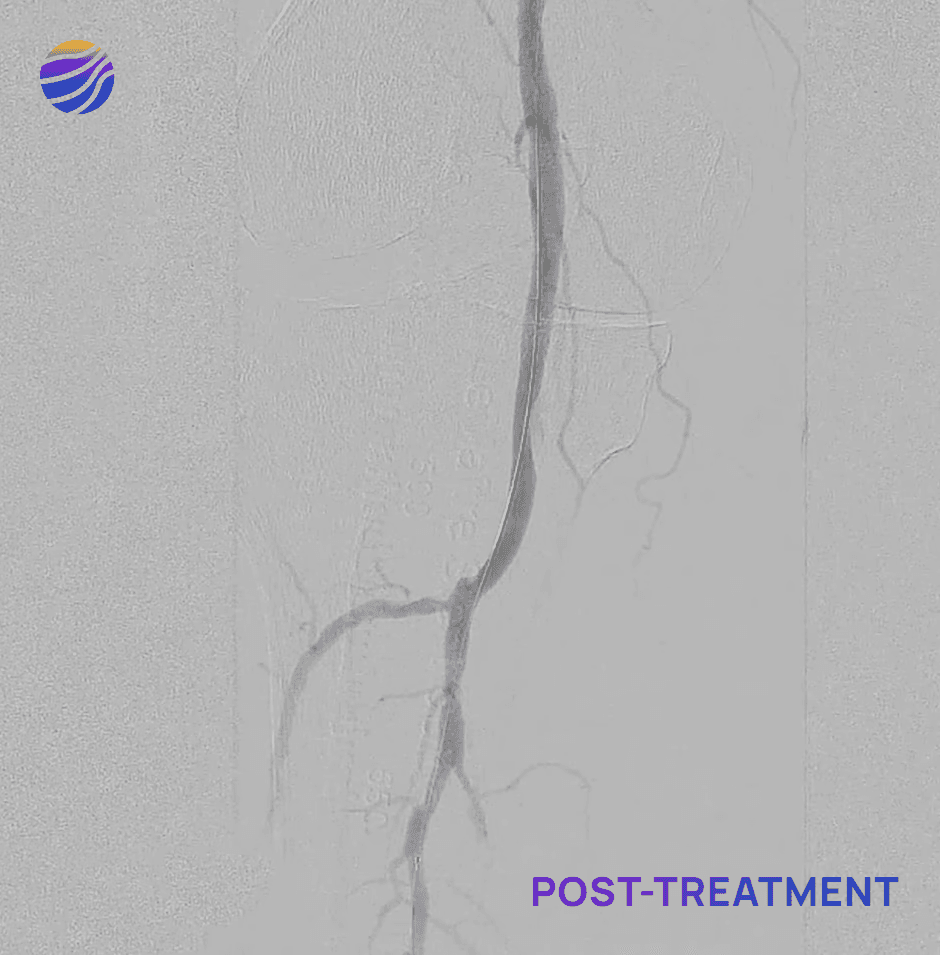
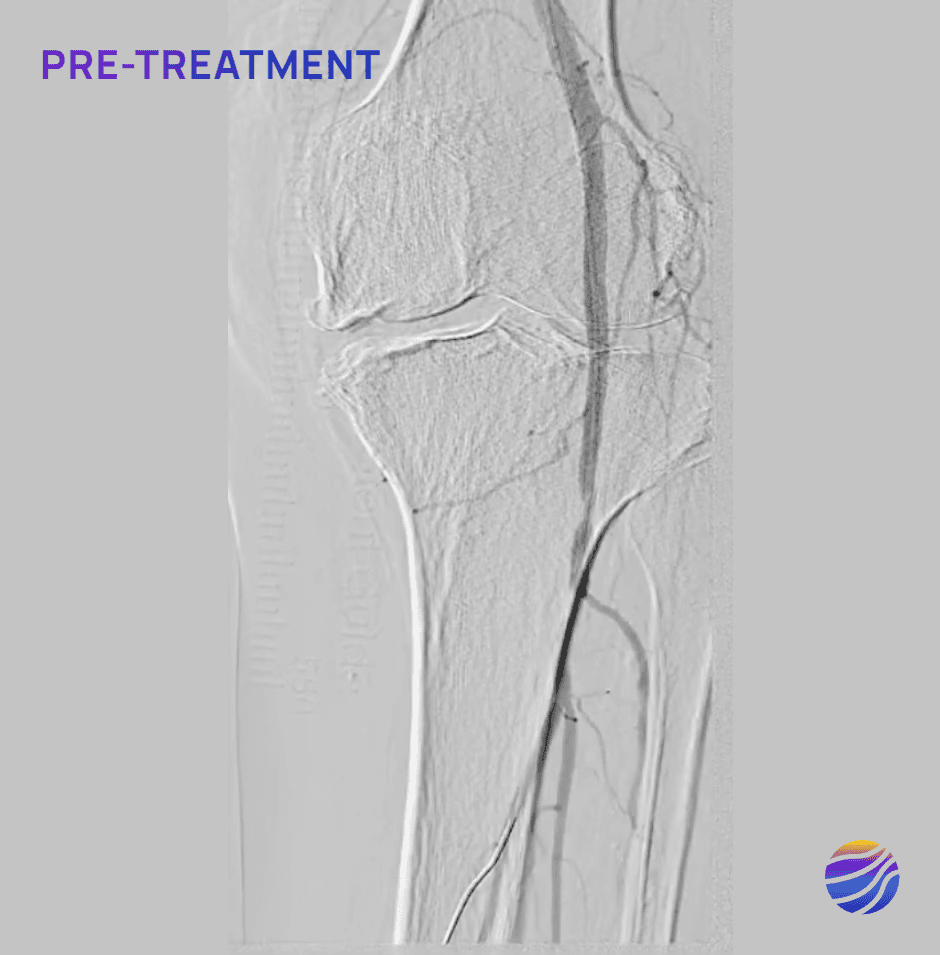
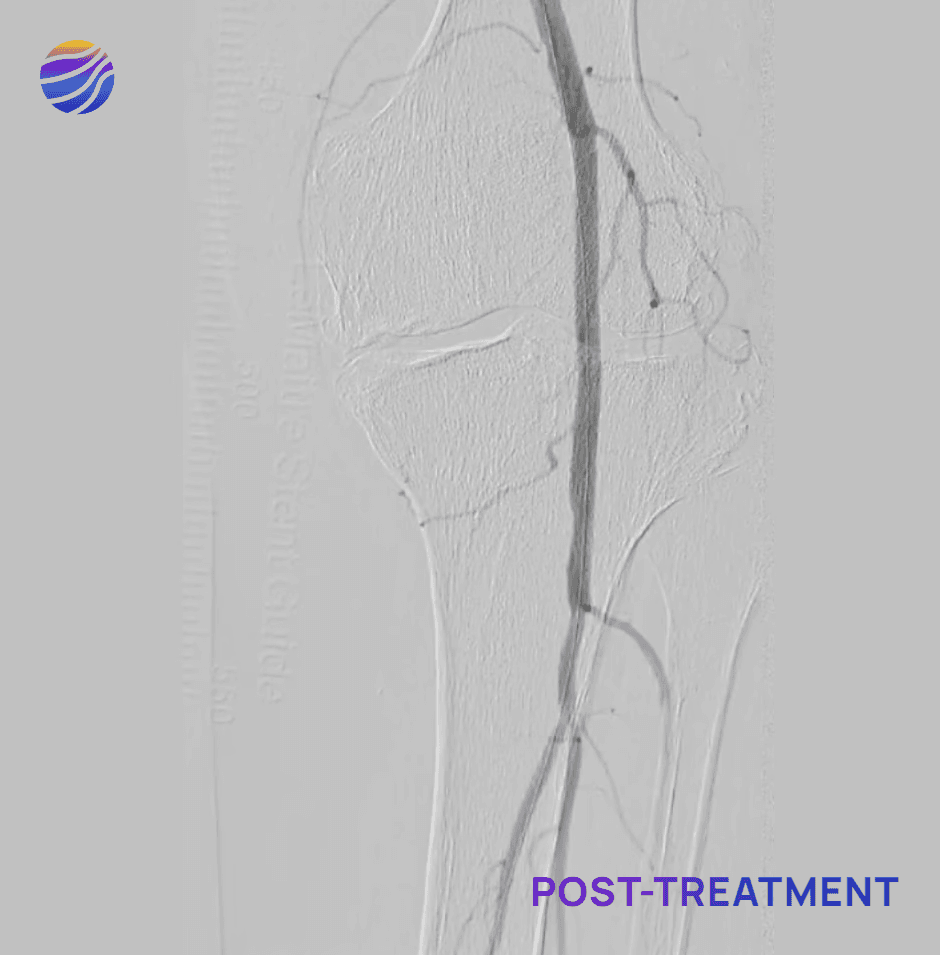
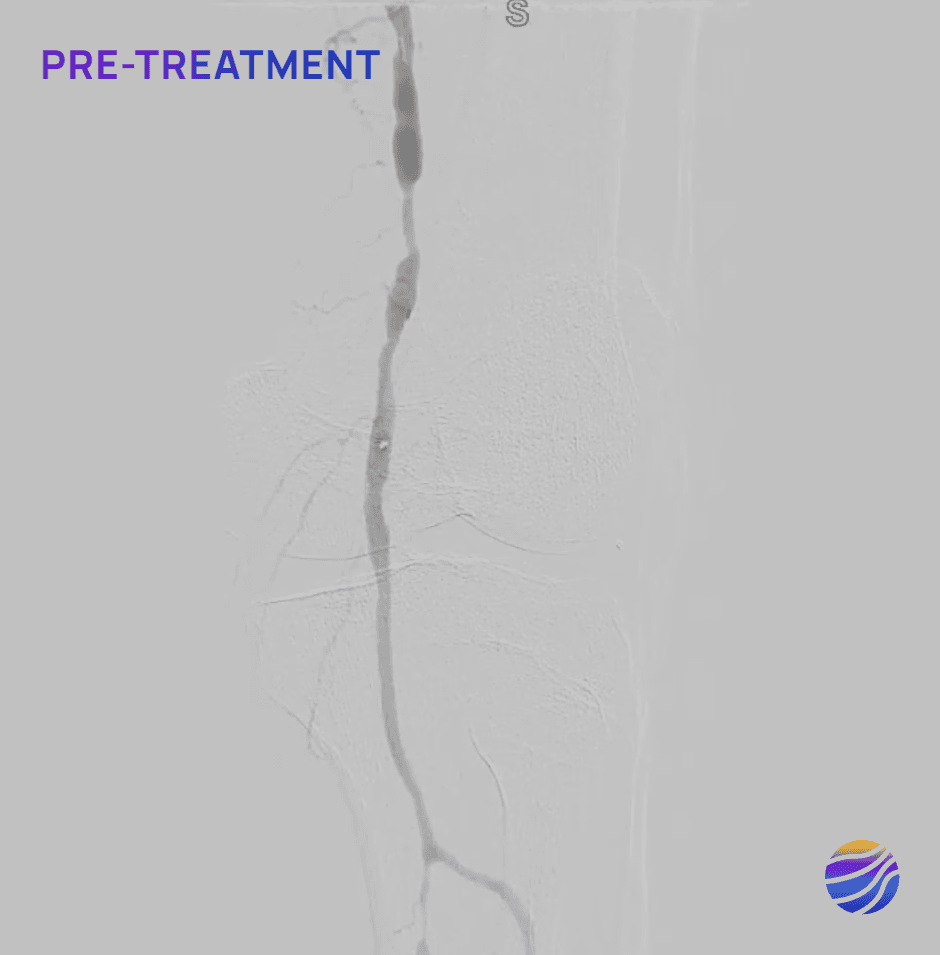
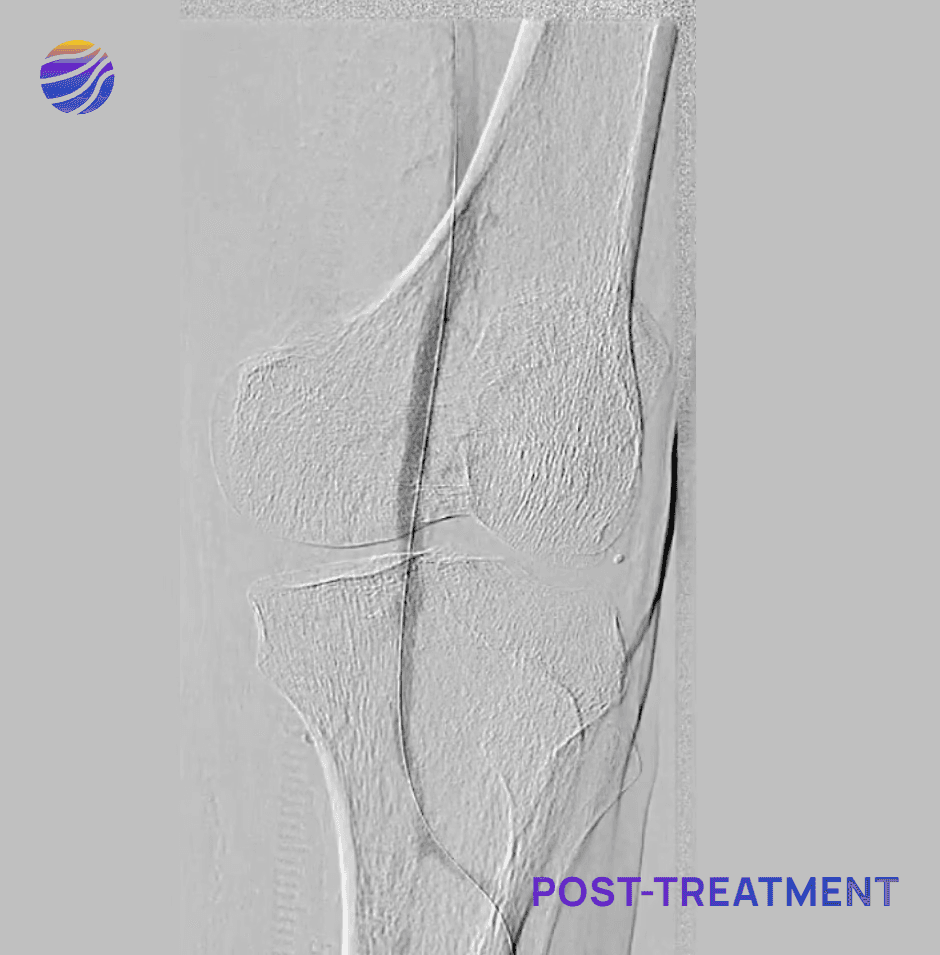
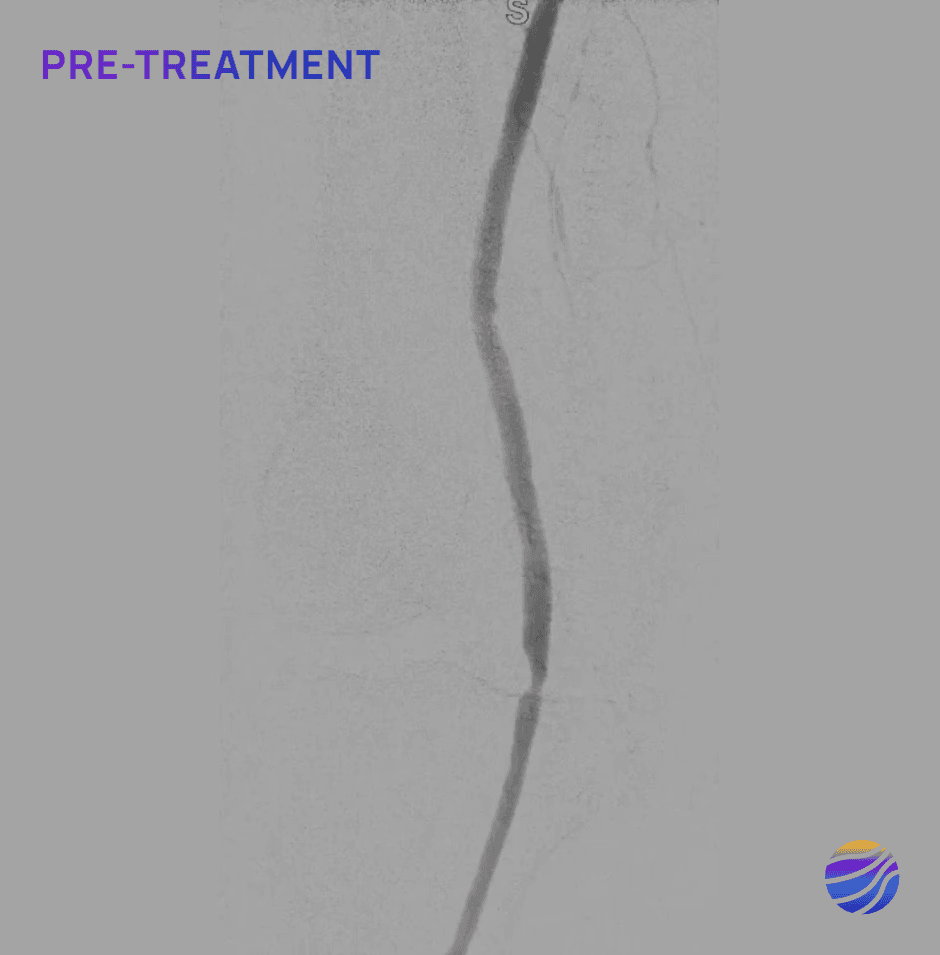
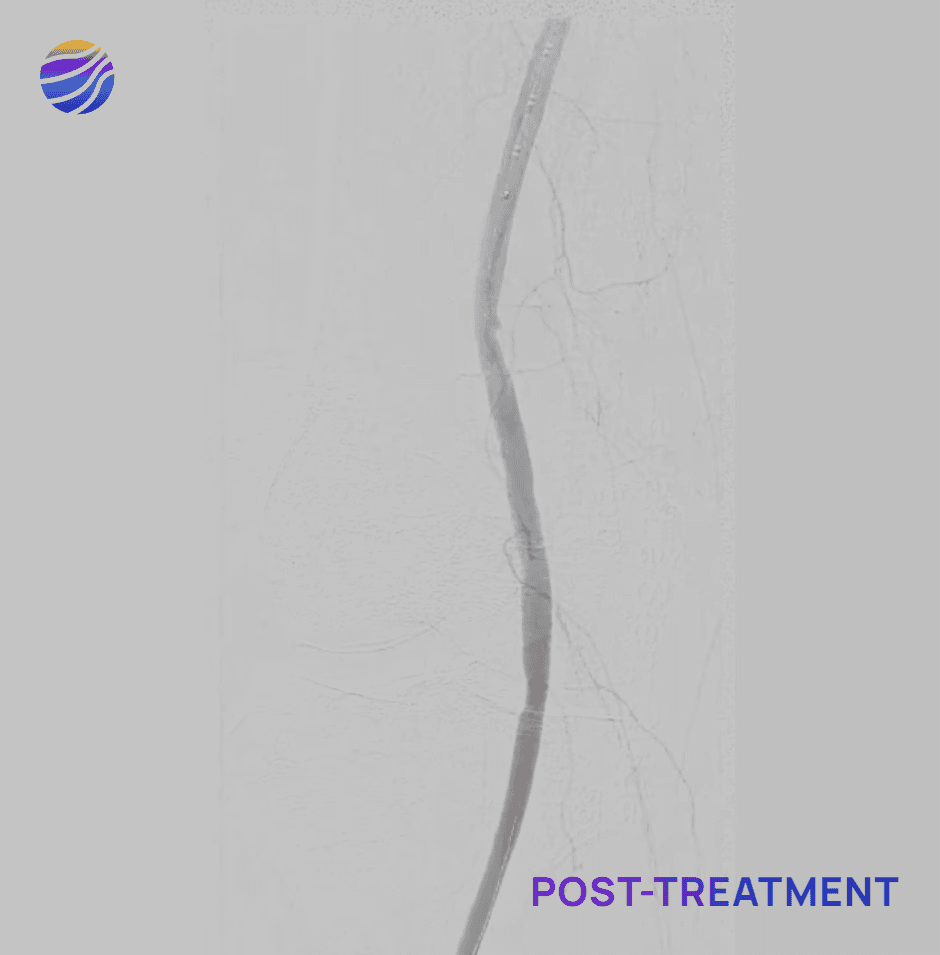
Selected FIH Case Studies
Subject 01
68-year-old male, BMI of 30.3, and RCC 6
Lesion Locations: Fem-Pop
Target Lesions: 3
IVL Pulses Delivered: 300
Total Lesion Length: 60mm
Results: <10% RDS
Subject 04
84-year-old female, BMI of 28.6, and RCC 4
Lesion Locations: Popliteal
Target Lesions: 1
IVL Pulses Delivered: 120
Total Lesion Length: 40mm
Results: 0% RDS
Subject 05
89-year-old male, BMI of 22.2, and RCC 4
Lesion Locations: Femoral
Target Lesions: 2
IVL Pulses Delivered: 90
Total Lesion Length: 160mm
Results: <10% RDS
Subject 08
71-year-old male, BMI of 27.4, and RCC 3
Lesion Locations: Fem-Pop
Target Lesions: 2
IVL Pulses Delivered: 300
Total Lesion Length: 40mm
Results: <10% RDS
Future Studies
Peripheral Pivotal IDE Trial
We anticipate IDE approval from FDA to begin our peripheral pivotal trial in the first half of 2025. This study will evaluate the safety and efficacy of our peripheral IVL system in treating patients with severely calcified peripheral arterial disease.